Science >Chemistry >Molecule and Molecular Mass > Calculation of Mass of a Molecule and an Atom
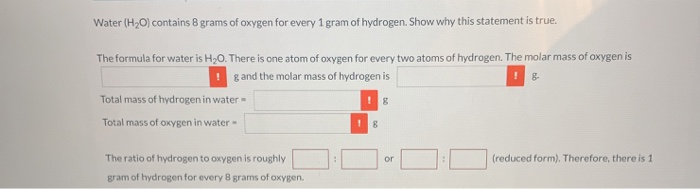
Atomic mass is a characteristic of an atom that is a measure of its size. It is plays a major role in the chemical properties of elements. This article discusses atomic mass and how it is calculated. A molecule of water has the chemical formula H 2 O, so it contains two hydrogen (H) atoms and one oxygen (O) atom. Hydrogen has an average atomic mass of 1.00794 amu. Oxygen atoms have an average mass of 15.9994 amu. The average mass of a molecule of H 2 O equals (1.00794)(2) + 15.9994 = 18.01528 amu, equivalent to 18.01528 g/mol. 1 mol oxygen atom contains 6. 0 2 3 × 1 0 2 3 = 1 6 g Mass of ⇒ 6. 0 2 3 × 1 0 2 3 oxygen atoms = 1 6 g Mass of 1 atom of oxygen = 6. 0 2 3 × 1 0 2 3 1 6 g = 2. 6 6 × 1 0 − 2 3 g. 6.02 x 10^23 atoms of oxygen has a mass of 15.999 grams. 1 atom of oxygen has an average mass of 15.99 atomic mass units. To find the number of grams of 1 atom of oxygen divide 15.999 g by Avogadro’s number.
In this article, we shall study the calculation of the mass of molecule and an atom.
Schematic Diagram for Mole Calculations:
Where, m = Given mass, M = Molar mass
v = Given volume, V = Molar volume = 22.4 dm3
n = Number of moles = m/M
Number of atoms = Number of molecules × Atomicity
Conversions:
To Calculate Mass of Given Moles:
Calculate the mass of the following.
2.5 moles of water:
Molecular mass of water (H2O) = 1 × 2 + 16 × 1 = 2 + 16 = 18 g
Number of moles of water = 2.5 = n
Mass of water = n × Molar mass
Serial box for mac os. Mass of water = 2.5 × 18 = 45 g

1.2 moles of carbon dioxide
Molecular mass of carbon dioxide (CO2) = 12 × 1 + 16 × 2 = 12 + 32 = 44 g
Number of moles of carbon dioxide = 1.2
Mass of carbon dioxide = n × Molar mass
Mass of carbon dioxide = 1.2 × 44 = 52.8 g
0.25 moles of sulphuric acid

Molecular mass of sulphuric acid (H2SO4) = 1 x 2 + 32 x 1 + 16 x 4 = 2 + 32 + 64 = 98 g
Number of moles of sulphuric acid = 0.25 = n
Mass of sulphuric acid = n × Molar mass
Mass of sulphuric acid = 0.25 × 98 = 24.5 g
0.1 moles of ammonia
Molecular mass of ammonia (NH3) = 14 x 1 + 1 x 3 = 14 + 3 = 17 g
Number of moles of ammonia = 0.1 = n
Mass of ammonia = n × Molar mass
Mass of ammonia = 0.1 × 17 = 1.7 g
3.5 moles of methane

Molecular mass of methane (CH4) = 12 x 1 + 1 x 4 = 12 + 4 = 16 g
Number of moles of methane = 3.5 = n
Mass of methane = n × Molar mass
Mass of methane = 3.5 × 16 = 56 g
2.4 moles of sulphur dioxide
Mass Of Oxygen In Kg
Molecular mass of sulphur dioxide (SO2) = 32 x 1 + 16 x 2 = 32 + 32 = 64 g
Number of moles of sulphur dioxide = 2.4 = n
Mass of sulphur dioxide = n × Molar mass
Mass of sulphur dioxide = 2.4 × 64 = 153.6 g
0.6 moles of bromine
Molecular mass of bromine (Br2) = 40 x 2 = 80 g
Number of moles of bromine = 0.6 = n
Mass of bromine = n × Molar mass
Mass of bromine = 0.6 × 80 = 48 g
To Calculate Mass of a Molecule and Atom:
Calculate the following
mass of one oxygen atom and oxygen molecule in kg.
Molecular mass of oxygen (O2) = 16 x 2 = 32 g
1 mole of oxygen is 32 g = 32 x 10-3 kg
1 mole of a substance contains 6.022 x 1023 molecules
Mass of each molecule of oxygen = (32 x 10-3)/(6.022 x 1023)
= 5.314 x 10-26 kg
Atomicity of oxygen (O2) molecule is 2
Mass of each atom of oxygen = 5.314 x 10-26 /2 = 2.657 x 10-26 kg
mass of one calcium atom in kg.
Molecular mass of calcium (Ca) = 40 g
1 mole of calcium is 40 g = 40 x 10-3 kg
1 mole of a substance contains 6.022 x 1023 molecules
Mass of each molecule of calcium = (40 x 10-3)/(6.022 x 1023)
= 6.642 x 10-26 kg
Atomicity of calcium (Ca) molecule is 1
Mass of each atom of calcium = 6.642 x 10-26 /1 = 6.642 x 10-26 kg
mass of one nitrogen atom and nitrogen molecule in kg.
Molecular mass of nitrogen (N2) = 14 x 2 = 28 g
1 mole of nitrogen is 28 g = 28 x 10-3 kg
1 mole of a substance contains 6.022 x 1023 molecules
Mass of each molecule of nitrogen = (28 x 10-3)/(6.022 x 1023)
= 4.650 x 10-26 kg
Atomicity of nitrogen (N2) molecule is 2
Mass of each atom of nitrogen = 4.650 x 10-26 /2 = 2.325 x 10-26 kg
mass of one sulphur dioxide molecule in grams.
Molecular mass of sulphur dioxide (SO2) = 32 x 1 + 16 x 2 = 64 g
1 mole of sulphur dioxide is 64 g
1 mole of a substance contains 6.022 x 1023 molecules
Mass of each molecule of sulphur dioxide = (64)/(6.022 x 1023)
= 1.602 x 10-22 kg
mass of 100 molecules of water.
Molecular mass of water (H2O) = 1 x 2 + 16 x 1 = 18 g
Mass Of Oxygen Atom Kg
1 mole of water is 18 g = 18 x 10-3 kg
1 mole of a substance contains 6.022 x 1023 molecules
Mass of each molecule of water = (18 x 10-3)/(6.022 x 1023) = 2.989 x 10-26 kg
Mass of 100 molecules of water = 2.989 x 10-26 x 100 = 2.989 x 10-24 kg
Calculation of Volume at STP
Calculate the volume of following at STP.
8.5 x 10-4 kg of ammonia
Molecular mass of ammonia (NH3) = 14 x 1 + 1 x 3 = 14 + 3 = 17 g
= 17 x 10-3 kg
Mass Of One Atom Of Oxygen Is Class 9th
Number of moles of ammonia = given mass/ molecular mass
= (8.5 x 10-4)/(17 x 10-3) = 0.05
1 mol of a gas at STP occupies 22.4 dm3 by volume
Volume of ammonia = number of moles x 22.4
Volume of 8.5 x 10-4 kg of ammonia at STP = 0.05 x 22.4 = 1.12 dm3
3.5 x 10-3 kg of nitrogen
Molecular mass of nitrogen (N2) = 14 x 2 = 28 g = 28 x 10-3 kg
Number of moles of nitrogen = given mass/ molecular mass
= (3.5 x 10-3)/(28 x 10-3) = 0.125
1 mol of a gas at STP occupies 22.4 dm3 by volume
Volume of nitrogen= number of moles x 22.4
Volume of 3.5 x 10-3 kg of nitrogen at STP = 0.125 x 22.4 = 2.8 dm3
14 g of nitrogen
Molecular mass of nitrogen (N2) = 14 x 2 = 28 g = 28 x 10-3 kg
Number of moles of nitrogen = given mass/ molecular mass
= (14 x 10-3)/(28 x 10-3) = 0.5
1 mol of a gas at STP occupies 22.4 dm3 by volume
Volume of nitrogen= number of moles x 22.4
Volume of 3.5 x 10-3 kg of nitrogen at STP = 0.5 x 22.4 = 11.2 dm3
6.023 x 1022 molecules of ammonia
Number of moles of ammonia = Given molecules/ Avogadro’s number
Number of moles of ammonia = (6.023 x 1022)/(6.023 x 1023) = 0.1
1 mol of a gas at STP occupies 22.4 dm3 by volume
Volume of ammonia = number of moles x 22.4
Volume of 6.023 x 1022 molecules of ammonia at STP = 0.1 x 22.4 = 2.24 dm3
2.008 x 1023 molecules of SO2 at STP.
Number of moles of SO2 = Given molecules/ Avogadro’s number
Number of moles of SO2 = (2.008 x 1022)/(6.023 x 1023) = 0.3334
1 mol of a gas at STP occupies 22.4 dm3 by volume
Volume of SO2 = number of moles x 22.4
Volume of 2.008 x 1023 molecules of SO2 at STP = 0.3334 x 22.4 = 7.469 dm3
0.2 mole of sulphur dioxide.
1 mol of a gas at STP occupies 22.4 dm3 by volume
Volume of sulphur dioxide = number of moles x 22.4
Volume of 0.2 moles of sulphur dioxide at STP = 0.2 x 22.4 = 4.48 dm3
Science >Chemistry >Molecule and Molecular Mass > Calculation of Mass of a Molecule and an Atom
